Myelodysplastic neoplasms (MDS) are hematopoietic stem cell (HSC) disorders characterized by ineffective hematopoiesis, peripheral cytopenia, and increased tendency to leukemic transformation. Somatic mutations in spliceosome machinery occur in approx. 50% of MDS patients. Among these, up to 30% of MDS patients carry a mutation in SF3B1. According to the 2022 classification system, MDS-SF3B1 is now considered as separate MDS subtype. Mutated SF3B1 perturbs mitochondrial function, resulting in apoptosis and ineffective erythropoiesis. The most common mutation of SF3B1, K700E, accounts for 50% of the variants, with additional codons (such as 666 and 662) acting as hotspot sites. Different mutations seem to be associated with distinct clinicopathological features. However, the prognostic implication of different SF3B1 mutations in MDS remains controversial and needs further clarification. This study investigated differences in clinical and molecular features of MDS-SF3B1 with different types of SF3B1 mutations.
To investigate a direct impact of different point mutations in SF3B1, the study was based on four isogenic NALM6 cell lines, the wildtype (wt) and three cell lines, each with different SF3B1 mutation (CRISPR/Cas9-edited K700E, K666N, and H662Q). To understand phenotypic manifestations of the mutations in the disease, we also analyzed clinical and transcriptional characteristics of 146 lower-risk MDS patients. Using RNA-seq in the cell lines and CD34+ BM patient cells (17 patients with no mutation, 11 patients with K700E SF3B1 mutation, 5 patients with K666N/E SF3B1 mutation), we investigated effects of different SF3B1 mutations on RNA splicing events and gene expression levels.
Initially, outcome analysis confirmed the previously described observation that patients with a SF3B1 mutation have favorable outcomes compared to those with other splicing factor mutations. Further, the analysis showed that specific mutations in SF3B1 tend to lead to different prognoses; patients with K700E had longer survival compared to those with other SF3B1 mutations (mean progression-free survival was 64.8 vs. 29.1 months).
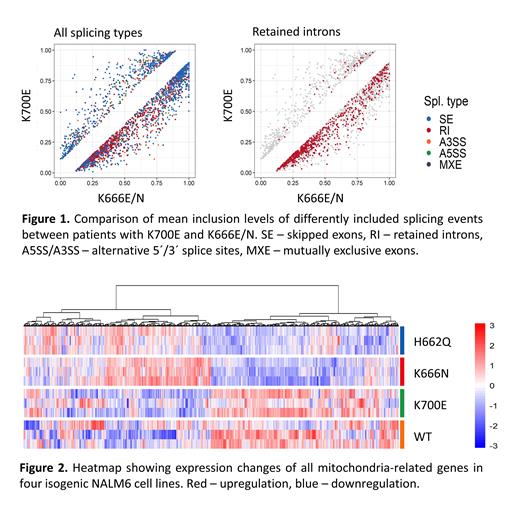
Analysis of RNA splicing in NALM6 showed that K666N and H662Q mutations share similar characteristics whereas K700E mutation is more similar to wt-cells. Most significant changes were found in retained introns (RI); increased RI inclusion levels were detected in K666N/H662Q cells compared to K700E/wt cells. In patients, RI events were also strongly increased in K666N/E compared to K700E samples (Fig. 1). A significant overlap was identified between cell line and patient data; out of 173 genes with higher RI inclusion level in K666N-NALM6 cells, 77 genes had higher RI inclusion also in patients. Interestingly, 12 out of these 77 genes were related to mitochondrial functions (e.g., CREBZF, DDX1, MRPL23, MUTYH, and RAF1).
RNA-seq data further showed significant alternations in gene expression levels in the four isogenic cell lines and also in patient samples with different mutations. Surprisingly, a more pronounced difference was detected in gene expression between patients with different SF3B1 mutations than between patients with no mutation and with any SF3B1 mutation (irrespective of its type), suggesting that specific SF3B1 mutations result in different phenotypes. Pathway analyses showed that deregulated genes in samples with K700E vs. other hotspot mutations were significantly enriched in electron transport, cell cycle, proliferation, quiescence, and HSC differentiation. Importantly, expression of multiple mitochondria-related genes was altered between K700E and K666N/H662Q NALM6 cells (334 genes, FDR < 0.01; Fig. 2), indicating different metabolic characteristics of the cells with non-K700E mutations. For example, several subunits of mitochondrial respiratory complexes I and IV were upregulated in K700E cells, pointing to differences in energetic metabolism.
Altogether, our data support the hypothesis that only K700E mutation of SF3B1 may have a positive prognostic value and that other SF3B1 mutations may influence cells differently, specifically affecting their mitochondrial functions. Based on our results, we conclude that prognostic implication of different SF3B1 mutations in MDS will need additional refinements in future.
Supported by AZV CR (NU20-03-00412), GA CR (20-19162S), and MH CZ-DRO (00023736).
Disclosures
Jonasova:AbbVie, BMS/Celgene, Novartis: Membership on an entity's Board of Directors or advisory committees; AbbVie, BMS/Celgene: Other: travel, accommodations, and expenses .

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal